Action levels in usp 1231 100cfu ml for purified water and 10cfu 100ml for water for injection are generally considered to represent a level above which the water is unfit for use.
Usp purified water vs wfi.
Difference between purified water and water for injection.
The procedure described below is designed for measuring the conductivity of purified water and water for injection.
The type of water for pharmaceutical use is determined by usp testing.
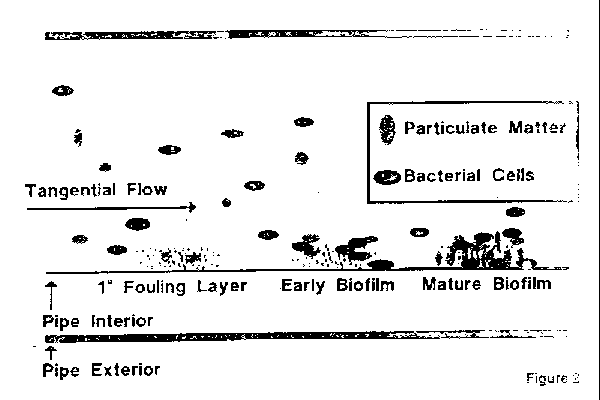
Unlike other official articles the bulk water monographs purified water and water for injection also limit how the article can be produced because of the belief that the nature and robustness of the purification process is directly related to the resulting purity.
100ml for wfi the microbiological test offers a good index of the level of contamination in a system.
The usp purified water and the usp wfi on the other hand are components or ingredient materials as they are termed by the usp intended to be used in the production of drug products.
Purified water and sterile purified water may be obtained by any suitable process.
As previously discussed because of the volume of water actually tested 1ml for endotoxins vs.
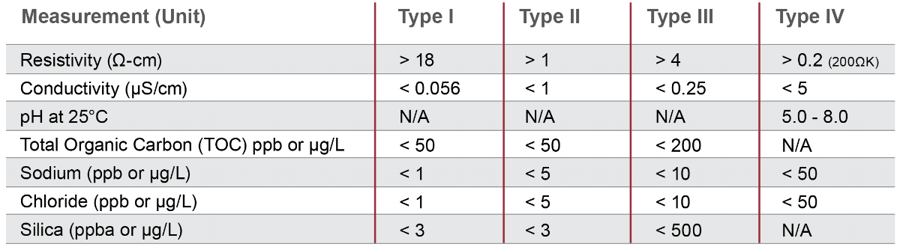
Wfi has stricter bacteria controls than purified water and hence the usp mandates these waters should have less than 500 ppb of total organic carbon fewer than 0 25 eu ml endotoxins and a conductivity of less than 1 3 us cm 25 c.
Distilled water water for injection sterile distilled water sterile wfi.
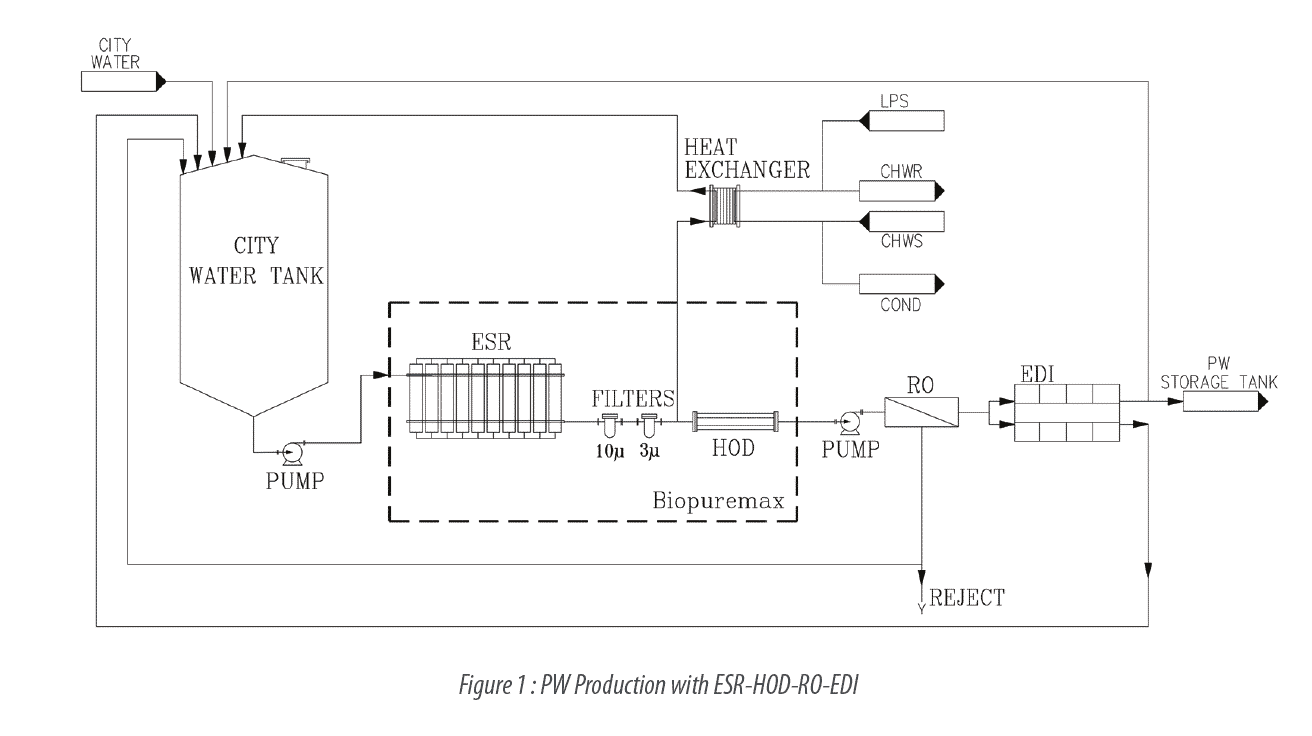
Water for injection is water purified by distillation or reverse osmosis.
Pharmaceutical technology is the independent source for information insight and analysis on bio pharmaceutical formulation development and manufacturing.
That is why an oos investigation must be undertaken if those action levels are exceeded.
From usp 1231 water for pharmaceutical purposes.
Sterile water for injection see the usp monograph is water for.
Specification as per usp.
The united states pharmacopeia usp defines this as a highly purified water containing less than 10 cfu 100 ml of aerobic bacteria.
Regulatory agencies have required that packaged pw and wfi be tested by the producer using toc and conductivity prior to packaging.